06-24-2024
Positive Phase 2 and Mechanism-of-Action Data for BOLD-100 Presented at 2024 Metals in Medicine Gordon Research Conference
Bold Therapeutics has been invited to speak at the 2024 Metals in Medicine Gordon Research Conference.

01-23-2024
Bold Therapeutics Announces Positive Phase 2 Safety and Efficacy Results for BOLD-100 in Advanced Metastatic Colorectal Cancer at ASCO GI 2024
Bold Therapeutics Announces Positive Phase 2 Safety and Efficacy Results for BOLD-100 in Advanced Metastatic Colorectal Cancer at ASCO GI 2024.

09-22-2023
Bold Therapeutics Selected as a 2023 Company to Watch by Life Sciences British Columbia (LSBC)
Bold Therapeutics Selected as a 2023 Company to Watch by Life Sciences British Columbia (LSBC).

06-07-2023
Bold Therapeutics Presents Positive Interim Phase 2 Results for BOLD-100 in Advanced Gastric and Biliary Tract Cancer at ASCO 2023
Bold Therapeutics announced positive interim results in advanced gastric and biliary tract cancer at the 2023 ASCO Annual Meeting.

04-18-2023
Bold Therapeutics Presents Jaw-Dropping Interim Phase 2 Clinical Data for BOLD-100 in the Treatment of Advanced Colorectal Cancer at AACR 2023
Bold Therapeutics is excited to share interim Phase 2 data for BOLD-100 in the treatment of advanced colorectal cancer.

04-13-2023
Bold Therapeutics to Present Best-in-Class Phase 2 Metastatic Colorectal Cancer Data at AACR 2023
Bold Therapeutics is excited to present robustly positive interim Phase 2 data in metastatic colorectal cancer at the upcoming AACR conference.
04-13-2023
Bold Therapeutics to Present Best-in-Class Phase 2 Metastatic Colorectal Cancer Data at AACR 2023
Bold Therapeutics is excited to present robustly positive interim Phase 2 data in metastatic colorectal cancer at the upcoming AACR conference.

10-14-2022
Bold Therapeutics Attending and Presenting at the BIO-Europe 2022 and BIO-Europe 2022 Virtual Conferences
Bold Therapeutics announces that they will be attending and presenting at BIO-Europe 2022 and BIO-Europe 2022 Virtual Conferences.

01-13-2022
Bold Therapeutics and Hana Pharm Extend Option Agreement for BOLD-100, a First-in-Class Anti-Resistance Therapeutic
Bold Therapeutics announces an extension of the option agreement with Hana Pharm for development and commercialization rights to BOLD-100 in South Korea.
09-16-2021
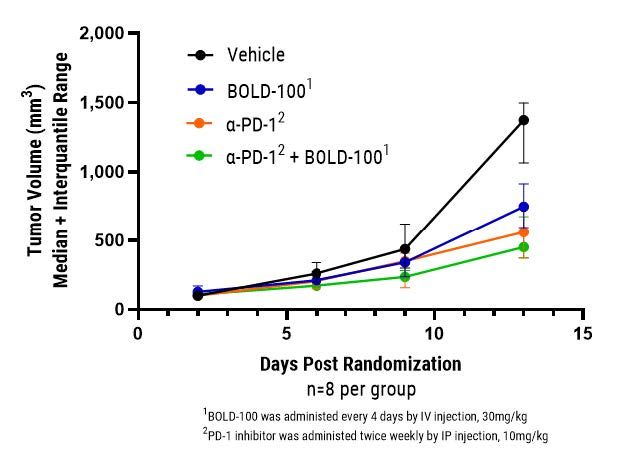
BOLD-100 Exhibits Potent Anti-Tumor Activity in Validated I/O In Vivo Models
Bold Therapeutics has demonstrated potent anti-tumor activity in combination with a PD-1 checkpoint inhibitor in an in vivo model of colorectal cancer.

07-07-2021
Bold Therapeutics Receives FDA Clearance to Add U.S. Sites for Phase 1b / 2a Trial of BOLD-100 in the Treatment of Advanced GI Cancers
Bold Therapeutics announces the addition of U.S. Sites for its phase 1b / 2a trial of BOLD-100 in the treatment of advanced GI cancers.
06-09-2020
Bold Therapeutics Receives Additional Funding to Support Development of BOLD-100 as a Novel Antiviral
Bold Therapeutics announces it is receiving additional funding of up to CAD$965,000 from NRC IRAP to support the development of BOLD-100 as a novel antiviral.

04-16-2021
BOLD-100 Effective In Vitro Against COVID-19 Variants
Bold Therapeutics announces that new research completed by collaborators shows BOLD-100 consistently reduced viral concentrations in COVID-19 variants.

04-09-2021
BOLD-100 MoA Research to Be Presented at the AACR Annual Meeting
Dr. Van Schaeybroeck will present research supporting BOLD-100 as a potential anti-resistance therapy for BRAF-mutant colorectal cancer at AACR annual meeting.
01-08-2021
Bold Therapeutics Secures Funding to Prepare BOLD-100 for COVID-19 Clinical Trials
Bold Therapeutics announces that it is one of four companies selected to receive funding from the National Research Council of Canada for its COVID-19 research.

12-18-2020
Bold Therapeutics COVID-19 Program Chosen by National Research Council of Canada for Development Support
Bold Therapeutics BOLD-100 program was one of only four COVID-19 therapeutics programs selected by the National Research Council for development support.

10-09-2020
Bold Therapeutics Successfully Initiates Clinical Trial of First-in-Class Anti-Cancer Agent BOLD-100
Bold Therapeutics announced that the first patient for the company�s Phase 1b clinical trial has been enrolled at the Cross Cancer Institute.

09-17-2020
BOLD-100 Shows Inhibition of DNA Repair Pathways in Breast Cancer
Georgetown University has recently published a new manuscript showing that Bold Therapeutics� BOLD-100 has anti-cancer potential in breast cancer.

08-17-2020
BOLD-100 Significantly Outperforms Remdesivir Head-to-Head in SARS-CoV-2 (COVID-19) Model
Bold Therapeutics recently generated additional data supporting rapid clinical development of BOLD-100 as a novel antiviral.

07-15-2020
BOLD-100 Demonstrates Nanomolar Range Inhibition of Live COVID-19 In Vitro
Bold Therapeutics has in vitro data showing that its lead clinical-stage therapeutic, BOLD-100, successfully inhibits live SARS-CoV-2 (COVID-19).

06-11-2020
Bold Therapeutics Expands COVID-19 Consortium
Bold Therapeutics is excited to expand its COVID-19 consortium to include four additional collaborators from prominent institutions in Canada and the U.K.

05-28-2020
Bold Therapeutics and Hana Pharm Execute Option Agreement for Exclusive Development and Commercialization Rights to BOLD-100 in South Korea
Bold Therapeutics announced it has executed an option agreement with Hana Pharm Co. for BOLD-100 in South Korea.
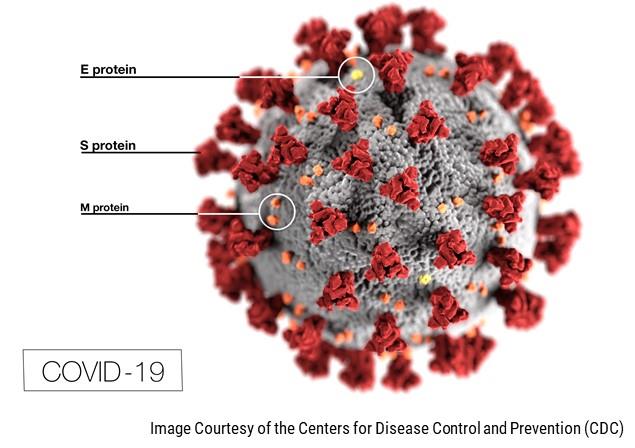
03-27-2020
Bold Therapeutics Seeks Collaborators for Rapid Development of BOLD-100; Lead Drug Candidate Has Potential Utility in the Treatment of COVID-19
Bold Therapeutics announced that recent developments have suggested the potential utility of its lead drug BOLD-100 as a novel antiviral agent.

03-27-2020
Bold Therapeutics to Initiate Phase 1b Trial of BOLD-100 in GI Cancers
Bold Therapeutics announced that it has received clearance from Health Canada to initiate a Phase 1b trial.